Rules for drawing newman projections
Table of Contents
Table of Contents
If you’re a chemistry student, you’ve likely encountered the concept of Newman projections. Drawing Newman projections can be a tricky process, but it’s an essential skill for understanding the conformational changes that molecules undergo.
The Pain of Drawing Newman Projections
Have you ever struggled to visualize how a molecule looks in three dimensions? Do you find yourself second-guessing the orientation of substituents and bonds? Drawing Newman projections can be a frustrating process, especially if you’re not familiar with the conventions and rules.
How to Draw Newman Projections
The first step in drawing a Newman projection is to identify the carbon-carbon bond that you want to focus on. Then, draw a vertical line to represent that bond, with one carbon on top and the other on the bottom.
Next, draw circles at the ends of each vertical line. These circles represent the carbon atoms that the bond connects.
Now you’re ready to add substituents to the carbon atoms. The convention is to draw the carbon on the left with its substituents projecting to the right, and the carbon on the right with its substituents projecting to the left.
Finally, you can label the substituents to keep track of which atoms are connected to which carbon.
Summary of How to Draw Newman Projections
The process of drawing Newman projections involves identifying the bond you want to focus on, drawing circles to represent the carbon atoms, adding substituents in the correct orientation, and labeling the substituents. Remember to follow the conventions of projecting substituents to opposite sides for each carbon.
My Experience with Drawing Newman Projections
When I first encountered Newman projections, I found them confusing and overwhelming. But with practice and guidance from my professor, I eventually became comfortable with the process. One tip that helped me was to draw the carbon-carbon bond and circles lightly first, and then add the substituents in stages to avoid confusion.
 Common Mistakes in Drawing Newman Projections
Common Mistakes in Drawing Newman Projections
One common mistake is projecting substituents on the same side of the carbon atom, rather than on opposite sides. This can result in incorrect stereochemistry and non-realistic structures. Another mistake is forgetting to label the substituents, which can make it difficult to keep track of which atoms are connected to which carbon.
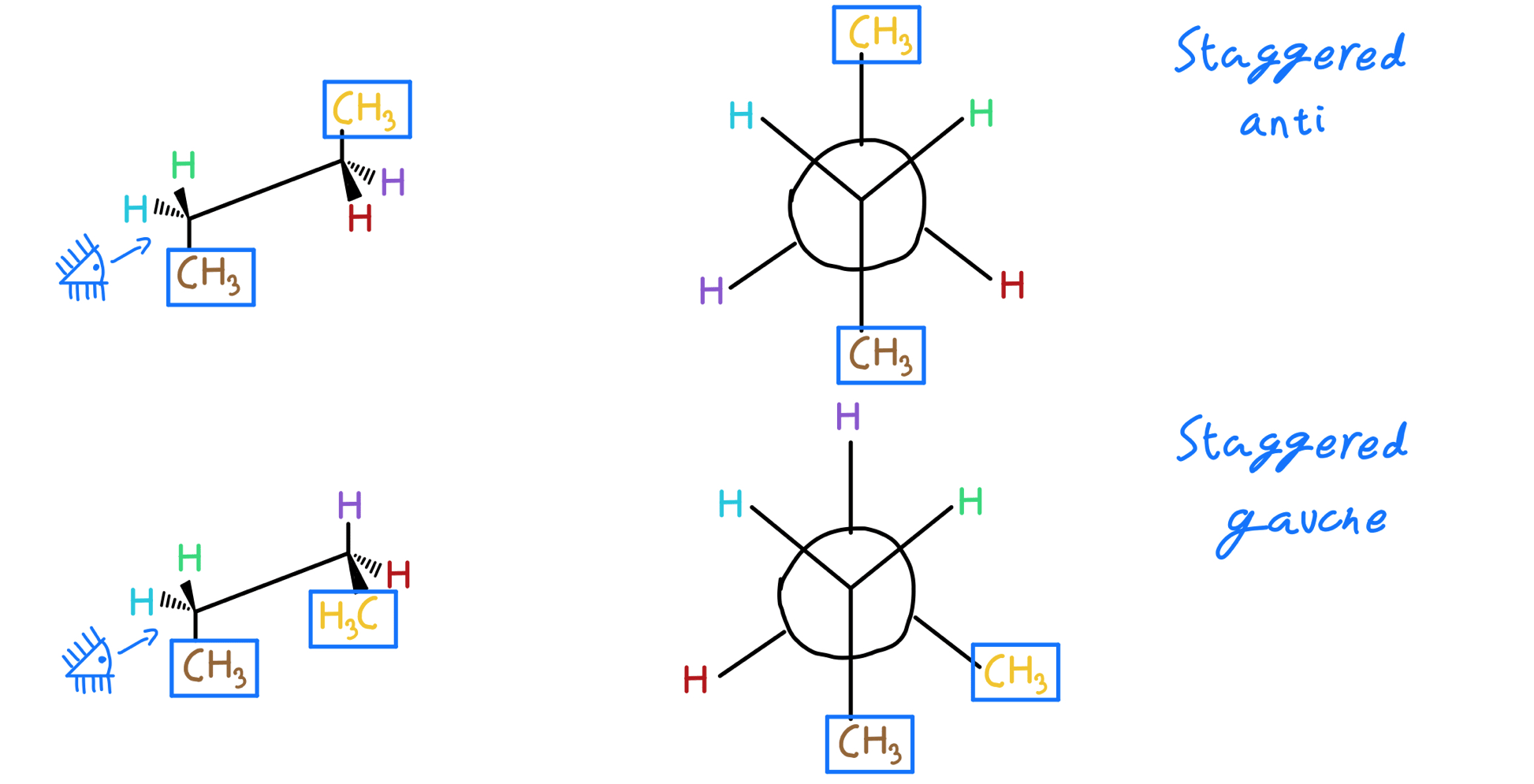 ### Going Deeper: Drawing Newman Projections of Alkanes
### Going Deeper: Drawing Newman Projections of Alkanes
When drawing Newman projections of alkanes, there are certain rules to follow. For example, staggered conformations are more stable than eclipsed conformations, and the anti-periplanar conformation is the most stable orientation for anti dihedral angles.
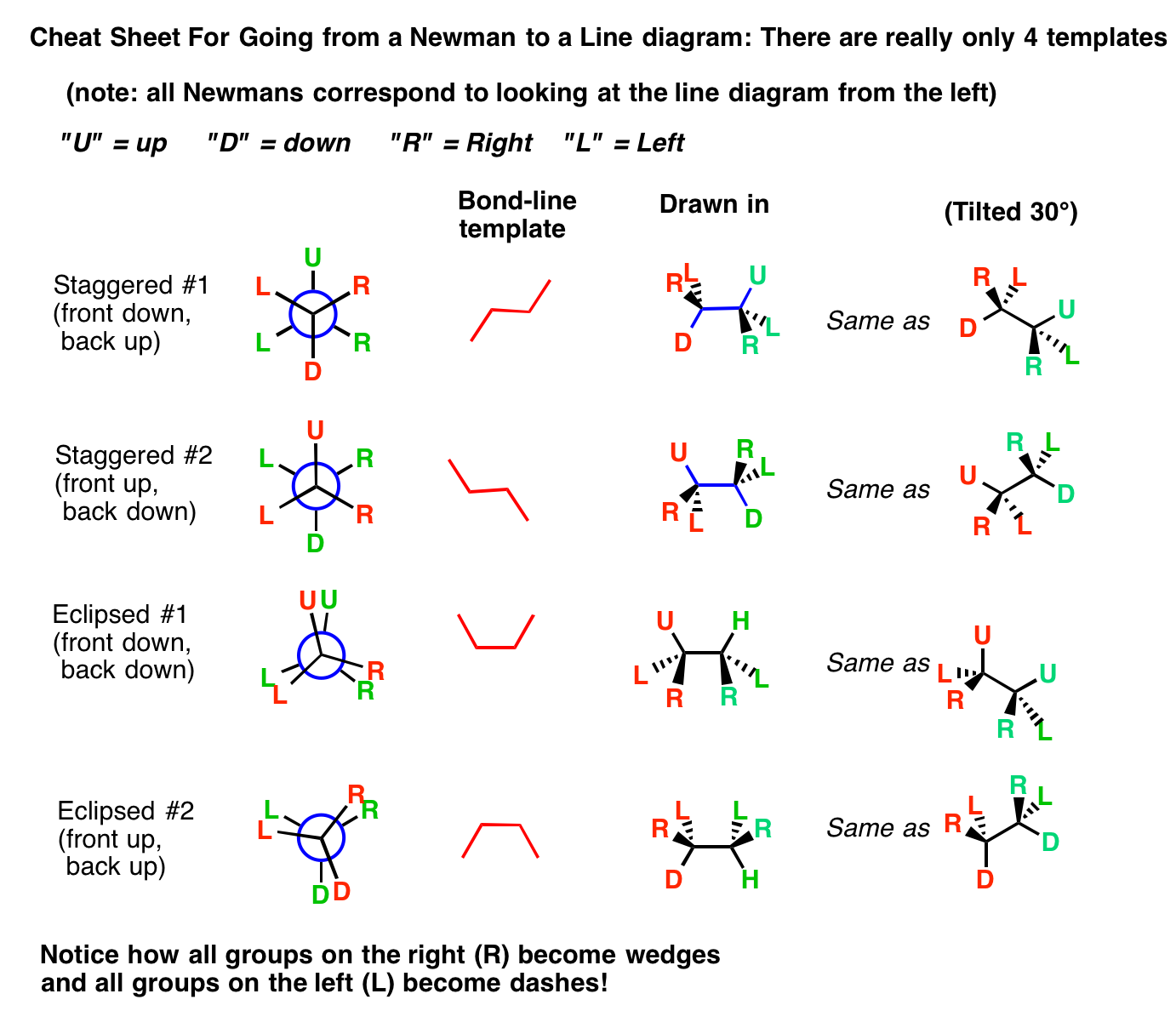 #### The Importance of Newman Projections
#### The Importance of Newman Projections
Newman projections are an essential tool for visualizing the three-dimensional structure of molecules, especially in the context of organic chemistry. By mastering the art of drawing Newman projections, you’ll be better equipped to understand the reactivity and properties of molecules.
Question and Answer Section: How to Draw Newman Projections
1. What is a Newman projection?
A Newman projection is a way of representing the three-dimensional structure of a molecule by looking down a specific bond. It shows the spatial orientation of substituents and atoms in the molecule.
2. Why are Newman projections important?
Newman projections are important for understanding the conformational changes that molecules undergo, as well as predicting the reactivity and properties of molecules.
3. Are there any rules for drawing Newman projections?
Yes, there are several rules to follow when drawing Newman projections. Some examples include projecting substituents on opposite sides of each carbon, drawing staggered conformations whenever possible, and using labels to identify each substituent.
4. What are some common mistakes in drawing Newman projections?
Some common mistakes include projecting substituents on the same side of the carbon, forgetting to label the substituents, and misinterpreting the spatial orientation of the molecule.
Conclusion of How to Draw Newman Projections
Drawing Newman projections takes practice and patience, but it’s an essential skill for any chemistry student or researcher. By following the conventions and rules, you can create accurate representations of complex molecules and better understand their properties and reactivity.
Gallery
Newman Projections | ChemTalk

Photo Credit by: bing.com / newman projections
Ch 3 - Understanding Diagrams

Photo Credit by: bing.com / newman wedged projection projections drawing propane chem hashed right colour groups code understanding ch03 ucalgary carey5th courses ca
Rules For Drawing Newman Projections
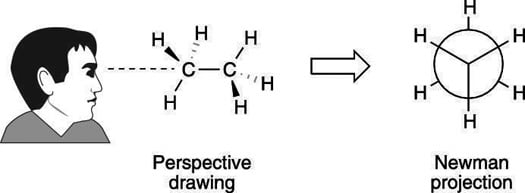
Photo Credit by: bing.com / newman projections interpret alkanes conformation
How Would You Draw Newman Projections Of Alkanes? | Socratic

Photo Credit by: bing.com / projections converting projection diagrams alkanes newmann socratic projeksjon hva assigning molecule conformers organisk kjemi
Rules For Drawing Newman Projections

Photo Credit by: bing.com / newman projections drawing chemistry organic prep clutch