Orbital molecular diagram mo oxygen o3 dioxygen o2 electrons c2 radical b2 configuration electron orbitals draw diagrams bonding energy level
Table of Contents
Table of Contents
Are you a chemistry student struggling to draw molecular orbital diagrams? Don’t worry, you’re not alone. Many students find this topic confusing and overwhelming, but with a little guidance, you can master the art of drawing molecular orbital diagrams.
The process of drawing molecular orbital diagrams can be challenging because it requires an understanding of atomic orbitals, electron configurations, and molecular bonding. It can be easy to get lost in the details and lose sight of the big picture. Additionally, there are different techniques to draw molecular orbital diagrams, which can add to the confusion.
The key to drawing molecular orbital diagrams is to break the process down into manageable steps. First, you need to determine the electron configuration of the atoms involved in the bonding. Next, you need to construct the molecular orbitals using a combination of atomic orbitals. Finally, you need to fill the molecular orbitals with the available electrons.
In summary, there are three main steps to drawing molecular orbital diagrams:
Step 1: Determine the electron configuration
Before you begin drawing the molecular orbital diagram, you need to determine the electron configuration of the atoms involved in the bonding. This will help you determine how many electrons each atom will contribute to the bonding process.
For example, if you are drawing the molecular orbital diagram for oxygen (O2), you would first need to determine the electron configuration of oxygen. Oxygen has an electron configuration of 1s2 2s2 2p4. This means that each oxygen atom has six valence electrons that can be used for bonding.
Step 2: Construct the molecular orbitals
Once you have determined the electron configuration of the atoms, you can begin constructing the molecular orbitals. To do this, you will need to combine the atomic orbitals of the atoms involved in the bonding process.
The combination of atomic orbitals can be represented using a molecular orbital diagram. This diagram shows the energy levels and relative positions of the atomic and molecular orbitals. The most important thing to remember when constructing the molecular orbitals is that the total number of molecular orbitals will be equal to the total number of atomic orbitals used in the combination.
Step 3: Fill the molecular orbitals
Once you have constructed the molecular orbitals, you can begin filling them with electrons. The Pauli exclusion principle states that no two electrons can occupy the same orbital with the same spin, so you will need to fill the orbitals one at a time, keeping the spins paired as much as possible.
When filling the molecular orbitals, you should start with the lowest energy level and work your way up. The electrons will fill the orbitals in order of increasing energy until all of the available electrons have been accounted for.
Question and Answer
Q: What is a molecular orbital diagram?
A: A molecular orbital diagram is a representation of the relative positions and energies of atomic and molecular orbitals in a molecule.
Q: Why is drawing molecular orbital diagrams important?
A: Drawing molecular orbital diagrams is important because it helps us understand the bonding and properties of molecules.
Q: What is the Pauli exclusion principle?
A: The Pauli exclusion principle states that no two electrons can occupy the same orbital with the same spin.
Q: How many steps are involved in drawing molecular orbital diagrams?
A: There are three main steps involved in drawing molecular orbital diagrams: determining the electron configuration, constructing the molecular orbitals, and filling the molecular orbitals with electrons.
Conclusion of How to Draw Molecular Orbital Diagram
Although drawing molecular orbital diagrams can be challenging, with a little practice and guidance, you can master this skill. By breaking the process down into manageable steps and understanding the underlying concepts, you can create accurate and informative diagrams that will help you understand the bonding and properties of molecules. Remember to start by determining the electron configuration, constructing the molecular orbitals, and filling the molecular orbitals with electrons. With these steps in mind, you’ll be well on your way to becoming a molecular orbital diagram pro!
Gallery
Mathematics - Origins Of Molecular Orbital Diagrams? - History Of

Photo Credit by: bing.com / orbital molecular diagrams does origins molecules chemistry mathematics description electrons questions
9.8: Second-Row Diatomic Molecules - Chemistry LibreTexts
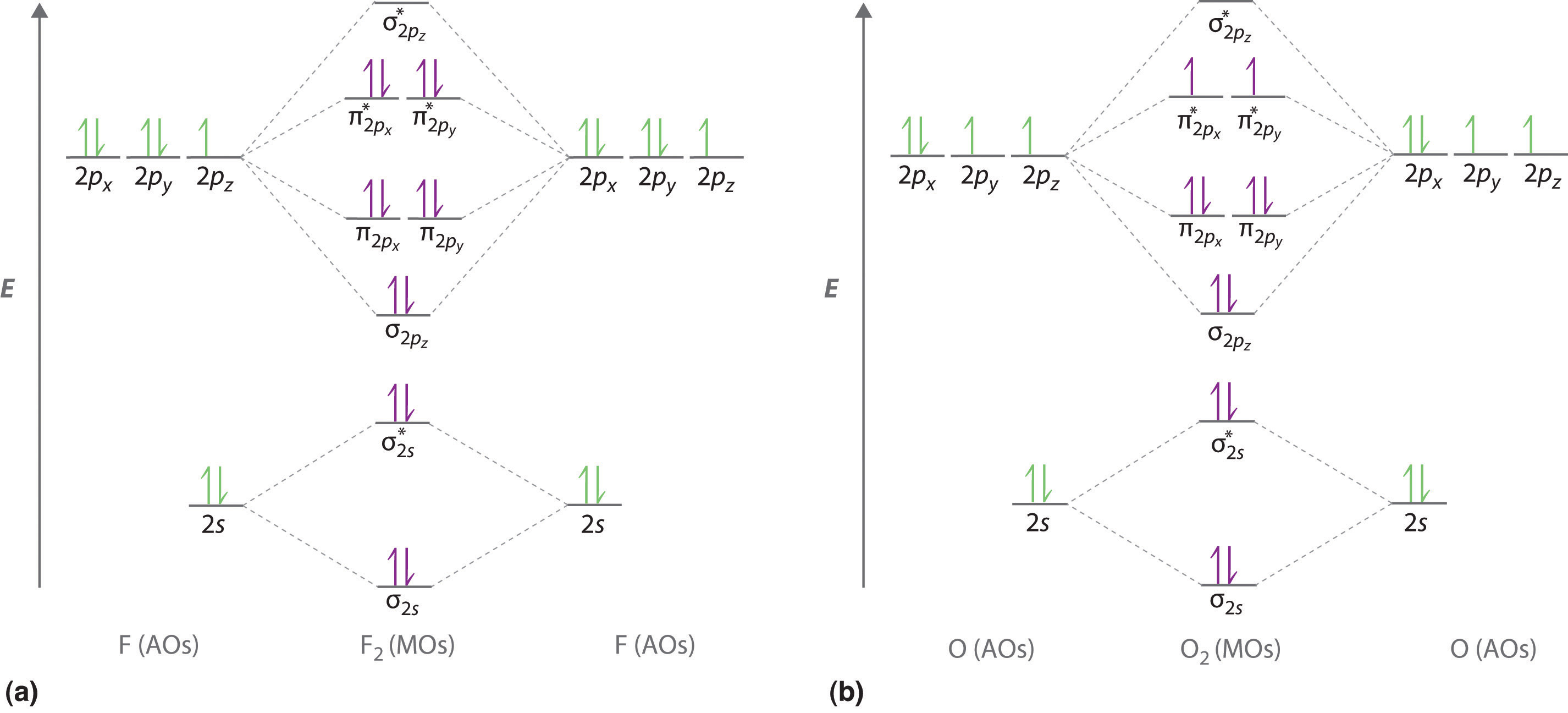
Photo Credit by: bing.com / orbital nh3 orbitals diatomic molecules of2 bonding delocalized libretexts chem homonuclear o2 valence electrons techiescientist hybridization pageindex conclusion
O3 Molecular Orbital Diagram

Photo Credit by: bing.com / orbital molecular diagram mo oxygen o3 dioxygen o2 electrons c2 radical b2 configuration electron orbitals draw diagrams bonding energy level
Use The Molecular Orbital Diagram Shown To Determine Which Of The

Photo Credit by: bing.com / orbital molecular diagrams determine simplified
PPT - Which One Of The Following Statements Is False ? PowerPoint
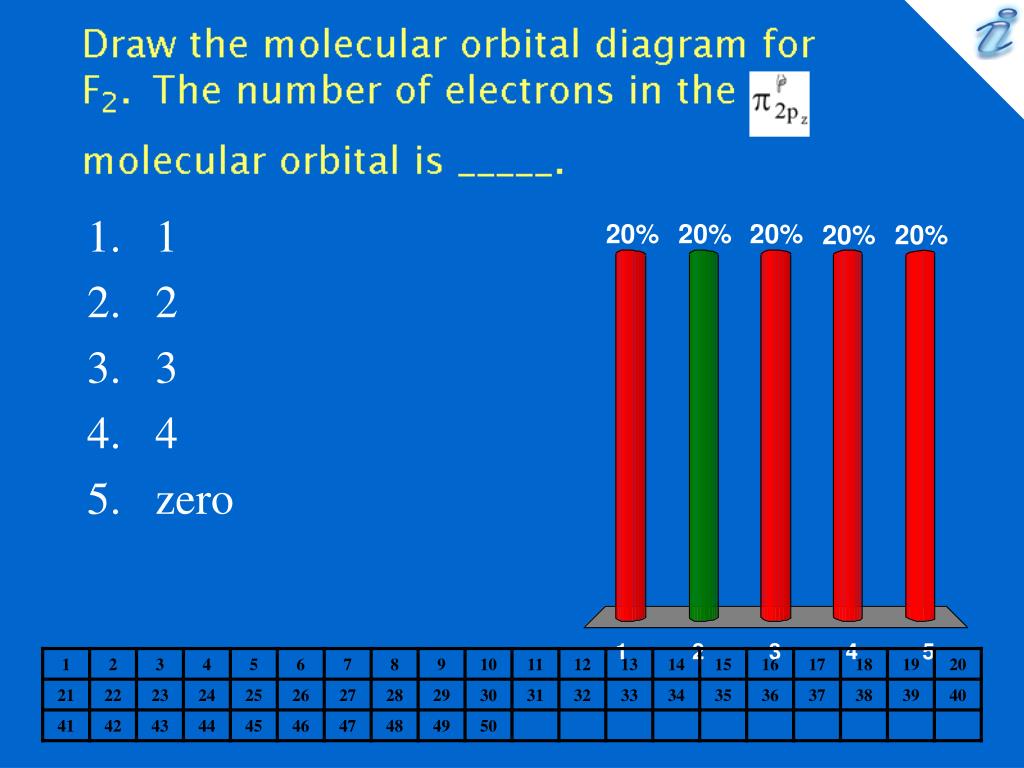
Photo Credit by: bing.com / orbital molecular electrons f2 number false statements following which ppt powerpoint presentation